Which of the Following Is Correct Regarding the Ph Scale
Ii It is the positive logarithm of H ion concentration of a given solution. Which of the following statement is correct regarding pH Scale.
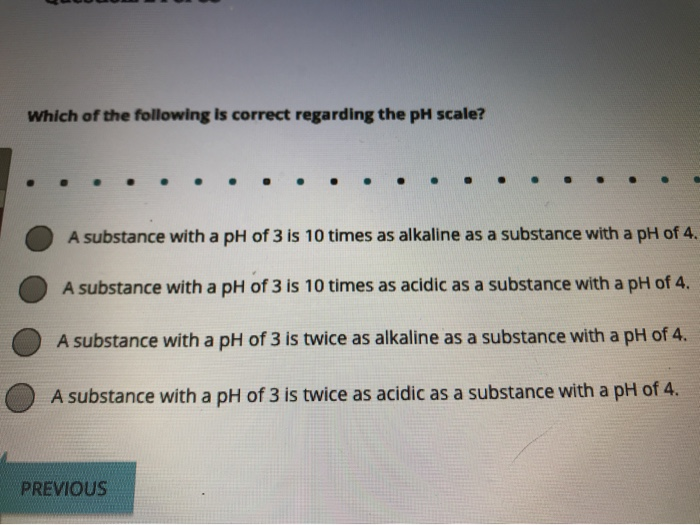
Solved Which Of The Following Is Correct Regarding The Ph Chegg Com
A substance with a pH of 3 is 10 times as acidic as a substance with a pH of 4.

. The lower the Ph the more acidic the solution. An atom is composed of protons electrons and neutrons. Iii It is a 14 point scale.
Basic pH levels are less than 7. In a pH expression the hydronium ions H3O can be abbreviated simply as H. Bases which have higher concentrations of hydronium ions than acids are found from 0 and up to 7.
Which of the following is correct regarding the pH scale. The pH of solutions inside most cells is close to 90. It is used by geologists paleontologists and other Earth scientists to describe the timing and relationships of events in geologic history.
The higher the numbers are the more basic the solution is and vise versa the lower the numbers are the more acidic the solution is. I It is the negative logarithm of H ion concentration of a given solution. Which of the following is correct regarding the pH scale.
Hydrogen is the only atom that doesnt have a neutron. Biology questions and answers. Which of the following is the incorrect statement for PH3.
The lower the whole number on the scale the greater the H concentration. PH is a scale that indicates the acidity of a solution. A solution with a pH of 4 has twice the H of a solution with a pH of 2.
Which of the following is the incorrect. C because the higher the Ph the more basic the solution. Which of the following statement is correct regarding pH Scale.
Which of the following statement is correct regarding pH Scale. When an acid and a base interact during a chemical reaction to produce water and a salt a type of reaction called an __________ reaction has occurred. According to the pH scale black coffee has a pH level of 5 meaning its neutral- in the middle.
A single unit change on the pH scale is equivalent to a 1 change in hydrogen ion concentration. The geologic time scale is a system of chronological dating that classifies geological strata in time. The p-Block Elements.
It is neither acidic nor basic and has a pH of 70. Pure water is neutral. I It is the negative logarithm of H ion concentration of a given solution.
Ii It is the positive logarithm of H ion concentration of a given solution. Ammonia is 11 and bleach is 13 so ammonia is. A substance with pH of 3 is 10 times as acidic as substance with PH of 4.
The correct options are B Electrons revolve around the nucleus C Nucleus is composed of neutrons and protons. Which of the following is the incorrect statement for. I It is the negative logarithm of H ion concentration of a given solution.
Iv pH is an example of an extrinsic property. Anything below 70 ranging from 00 to 69 is acidic and anything above 70 from 71 to 140 is alkaline. The blood in your veins is slightly alkaline pH 74.
The environment in your. Which of the following statement is correct regarding pH Scale. The pH scale is a measure of oxygen ion concentration.
A i and iii. The concentration of hydronium is represented by H3O and this value determines the pH of. Which of the following statements about pH is true.
Bases which have higher concentrations of hydronium ions than acids are found from 14 and down to 7. Ii It is the positive logarithm of H ion concentration of a given solution. Acids which have higher concentrations of hydronium ions than bases are found from 0 and up to 7.
A substance with a pH of 3 is twice as alkaline as a substance with a pH of 4. Which of the following statements about the pH scale is true. Iv pH is an example of an extrinsic property.
PH 0 is neutral. A substance with pH. Iii It is a 14 point scale.
Iii It is a 14 point scale. Which of the following is correct regarding the pH scale. Iv pH is an example of an extrinsic property.
I It is the negative logarithm of H ion concentration of a given solution. Which of the following is true regarding the pH scale. It would be choice C.
Both neutrons and protons are situated at the center of an atom and this dense region is called nucleus. A substance with a pH of 3 is 10 times as acidic as a substance with pH of 4 The main function of lymphatic circulation is to. An increase in hydrogen ion concentration means a decrease in pH scale units.
The pH scale is based on a scale involving powers of 10. In this situation we are focusing on specifically black coffee. Klondikegj and 29 more users found this answer helpful.
Correct options are A B and D p H is the negative logarithm of hydrogen ion concentration.
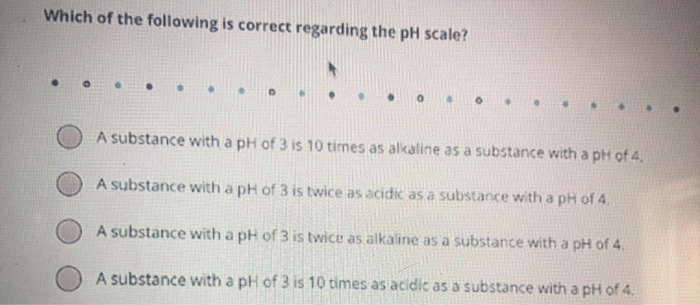
Solved Which Of The Following Is Correct Regarding The Ph Chegg Com
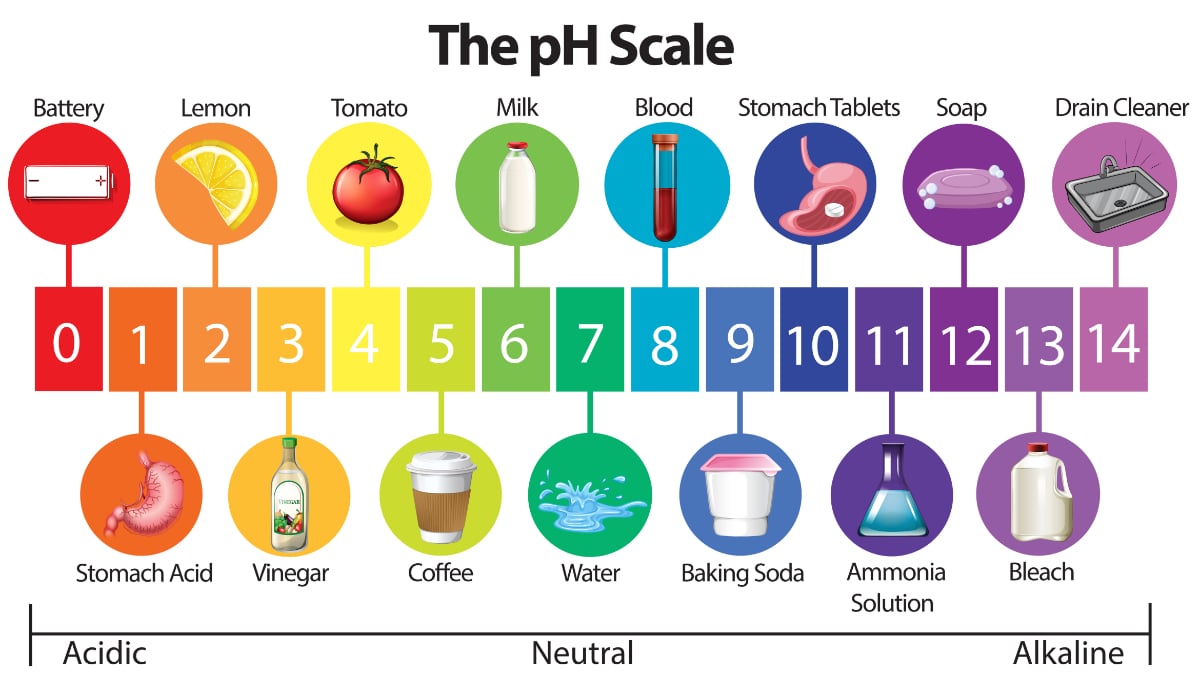
Water Quality 101 What Is Ph In Water Testing

Solved Which Of The Following Is Correct Regarding The Ph Chegg Com
0 Response to "Which of the Following Is Correct Regarding the Ph Scale"
Post a Comment